SARS-COV-2 ON-SITE DETECTION KIT FOR SURFACES
SURFACE TESTING FOR CORONAVIRUS MADE EASY
COV-Hygien Xpress test kits enable you to perform hygiene tests easily with no need for a laboratory. Testing with COV-Hygien Xpress kits minimizes start-up time, hands-on time, and time-to-result.
COV-HYGIEN XPRESS KITS ARE DESIGNED WITH SIMPLICITY IN MIND
Self-contained
All-in-one kit, with swabs, buffer, detections strips, tube, tube holder, labels, and marker
Ready-To-GO
No additional equipment necessary,
ready to use
Easy to Store And use
Long shelf-life at room temperature, easy-to-interpret results
Reliable RESULTS
- DO IT YOURSELF
- CHOOSING A TEST
- CHARACTERISTICS
- DOWNLOADS
THE SIMPLEST WAY TO TEST SURFACES FOR THE PRESENCE OF SARS-CoV-2
Do It Yourself!
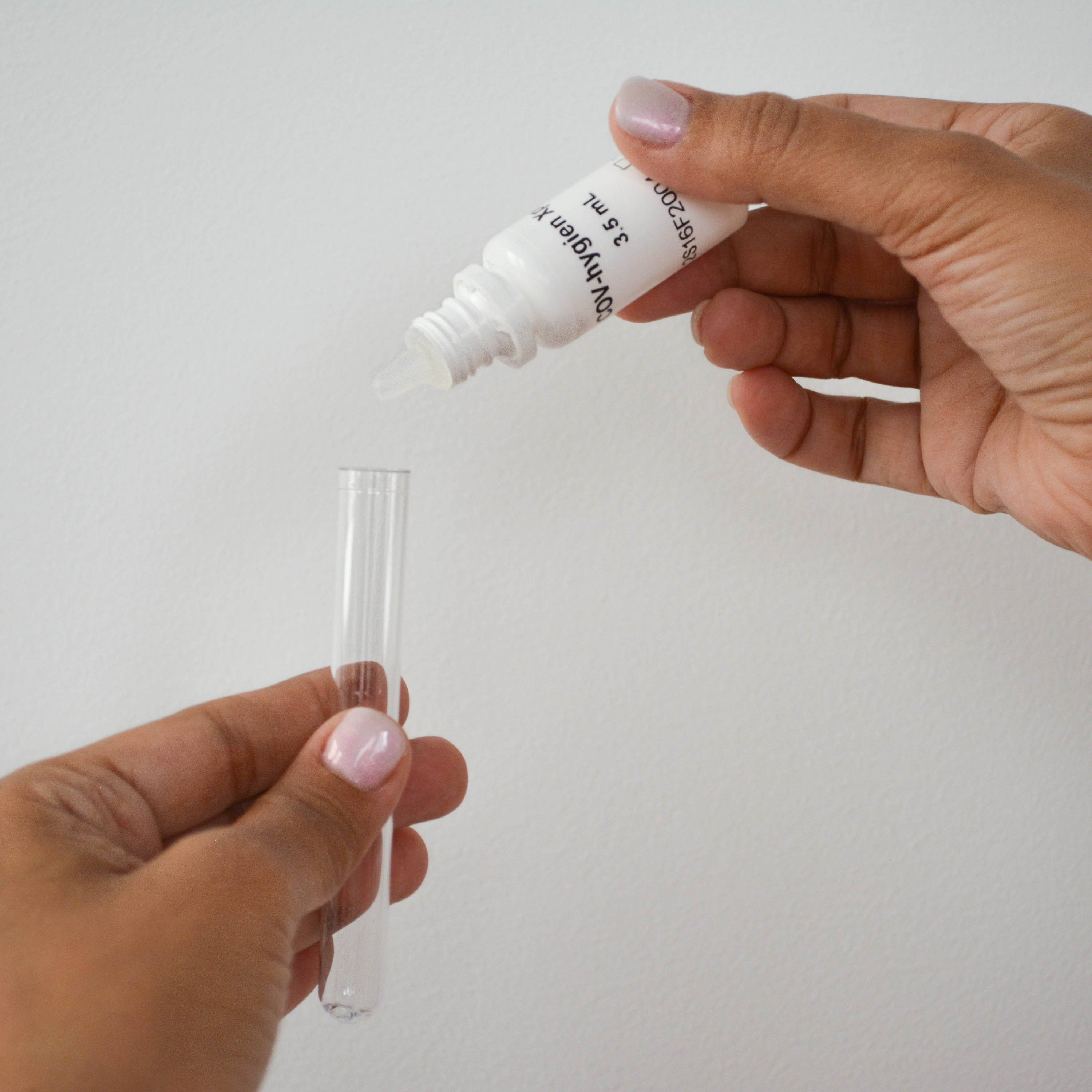

① Add 15 drops of reagent to the tube
② Soak the swab, then wring it out
③ Swab the surface you wish to test

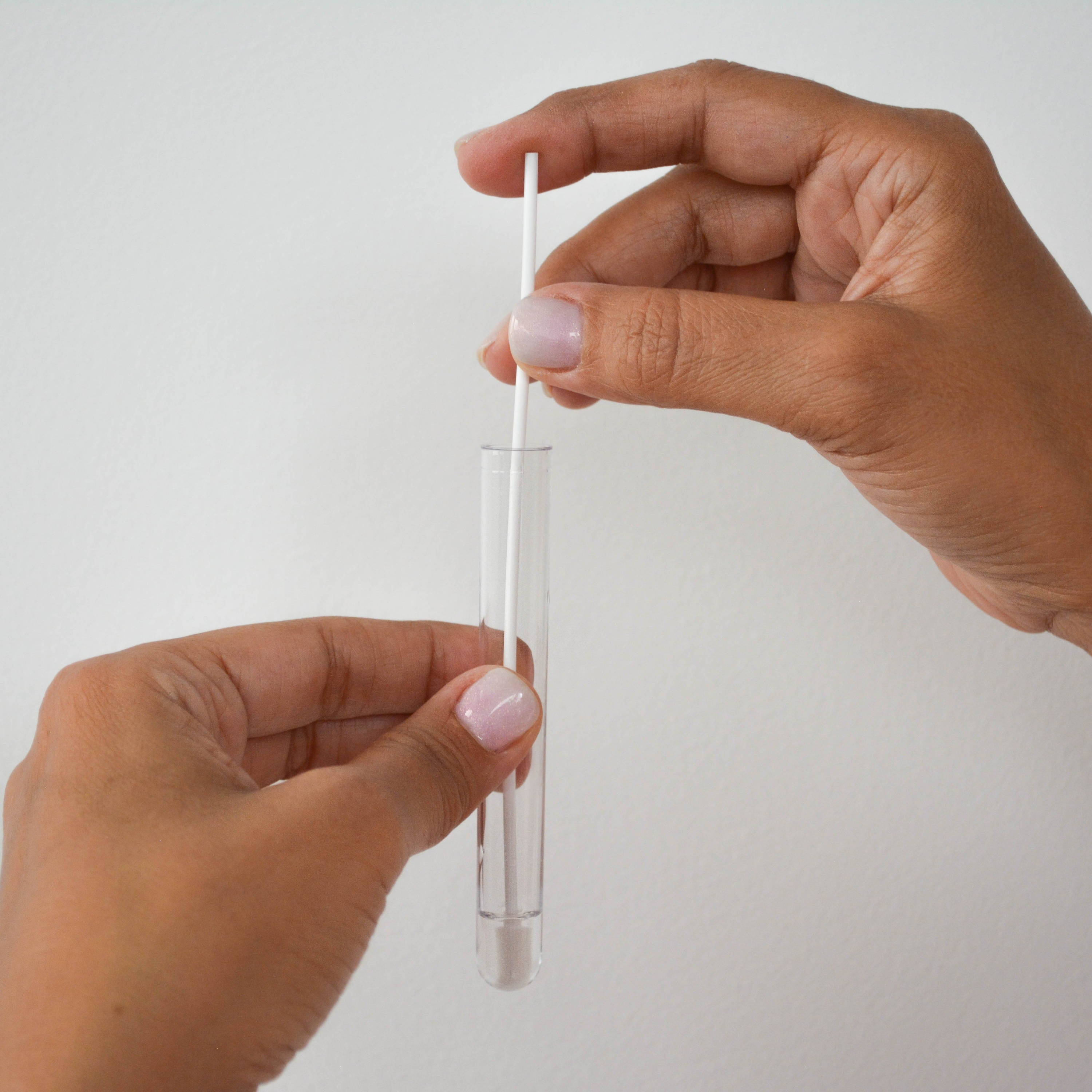
④ Stir the swab in the reagent & wring it out
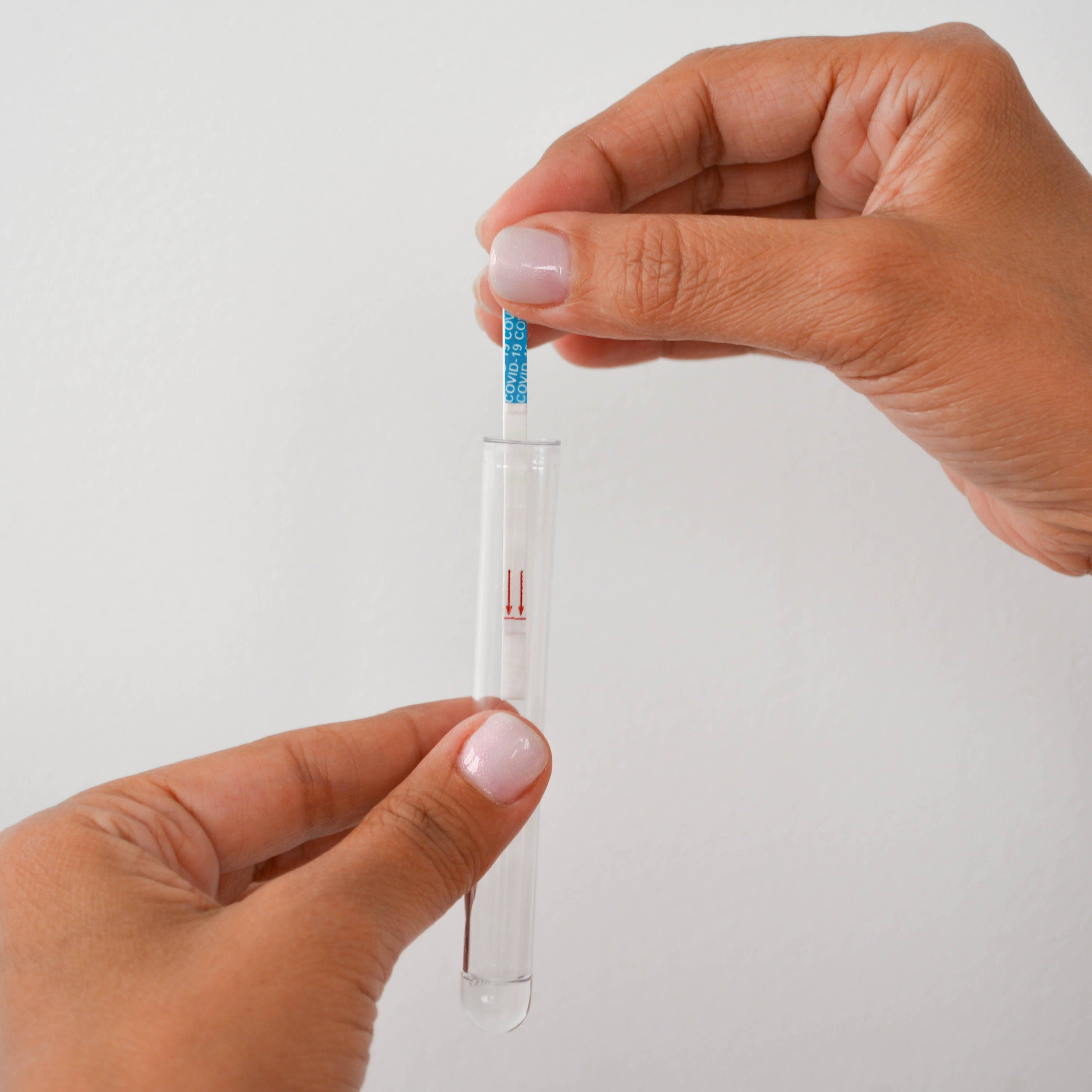
⑤ Insert a detection strip into the tube
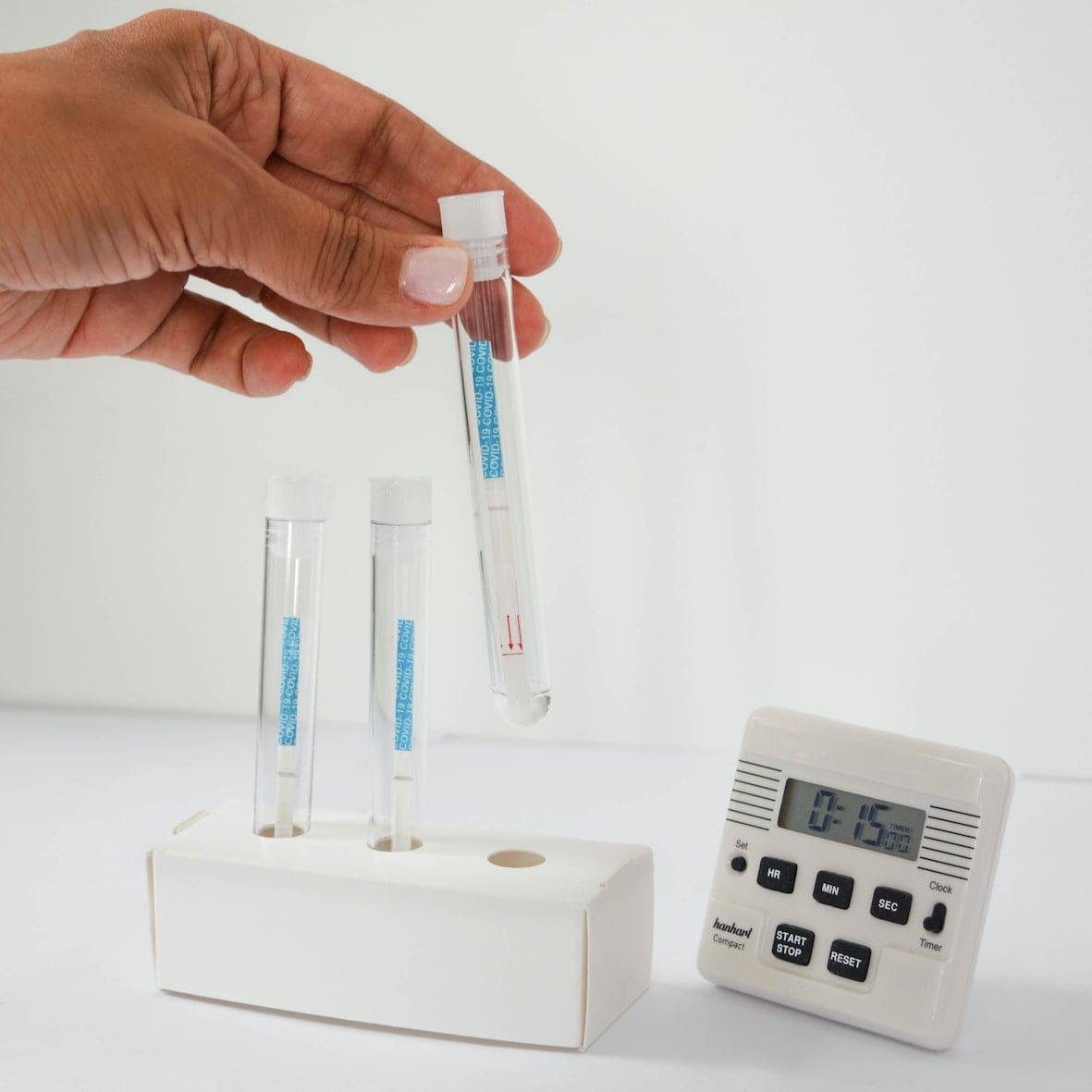
⑥ Read the results 15' later
NEGATIVE
POSITIVE
CHOOSING THE RIGHT TEST FOR DETECTING SURFACE CONTAMINATION
In hygiene monitoring, time-to-result is of essence because in case of a failed test, it allows for another cleaning and sanitation to take place before normal activities resume
" The COVID-19 pandemic has created a public health crisis. Because SARS-CoV-2 can spreadfrom individuals with pre-symptomatic, symptomatic, and asymptomatic infections, there-opening of societies and the control of virus spread will be facilitated by robust surveillance,for which virus testing will often be central. After infection, individuals undergo a period of incubationduring which viral titers are usually too low to detect, followed by an exponential growthof virus, leading to a peak viral load and infectiousness, and ending with declining viral levelsand clearance. Given the pattern of viral load kinetics, we model surveillance effectivenessconsidering test sensitivities, frequency, and sample-to-answer reporting time. These resultsdemonstrate that effective surveillance, including time to first detection and outbreak control, dependslargely on frequency of testing and the speed of reporting, and is only marginally improvedby high test sensitivity. We therefore conclude that surveillance should prioritize accessibility, frequency, and sample-to-answer time; analytical limits of detection should be secondary." *
The effectiveness of sanitization procedures is routinely monitored by assessing the quantity of microorganisms present, either using culture methods, which are quantitative and very informative but slow, or by measuring ATP, which is qualitative and less informative but rapid.
The main advantages of Immuno-Detection over PCR are true portability and simplicity: no equipment, electricity, or expertise is required.
Although both PCR and Immuno-Detection are rapid techniques that can be performed within minutes, PCR requires sophisticated equipment and expertise, and is often only available in a laboratory setting. Immuno-Detection tests in a laminar flow format, such as pregnancy test strips, can be carried out by virtually anyone, anywhere, under any conditions, and constitute a reliable indicator of the status of the person or location tested.
* Test sensitivity is secondary to frequency andturnaround time for COVID-19 surveillance
MedRxiv, posted June 27, 2020
Authors: Daniel B. Larremorey (1,2), Bryan Wilder (3), Evan Lester(6,5), Soraya Shehata (4,5),James M. Burke (6), James A. Hay (7,8), Milind Tambe (3), Michael J. Minaz (7,8,9), andRoy Parker (4,6,10,2)
(1) Department of Computer Science, University of Colorado Boulder, (2) BioFrontiers Institute, University of Colorado at Boulder (3) Center for Research on Computation & Society, Harvard John A Paulson School of Engineering andApplied Sciences, Harvard University (4) Department of Molecular, Cellular and Developmental Biology, University of Colorado (5) Medical Scientist Training Program, University of Colorado Anschutz Medical Campus (6) Department of Biochemistry, University of Colorado Boulder (7) Center for Communicable Disease Dynamics, Department of Epidemiology, Harvard T.H. Chan School ofPublic Health (8) Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health (9) Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School (10) Howard Hughes Medical Institute
COV-Hygien Xpress CHARACTERISTICS
INTENDED USE
This kit is designed to detect traces of SARS-CoV-2 on surfaces.
It is based on the detection of specific antigens.
All necessary reagents are provided.
WARNING
NOT for diagnostic use. For surface testing only.
PERFORMANCE CHARACTERISTICS of the Immuno-Detection Strip
Viral detection limit: 5x10E3 pfu/mL
During the development phase, the analytical performance of the assay was performed using a 2-fold serial dilution of the virus (in viral culture medium) in parallel with titrating the same virus preparation on Vero E6 cells (by plaque assay).
Results may vary with cell line, cell line preparation, culture media composition and in particular the presence of serum, as well as lysis conditions
Recombinant nucleocapsid (recN) protein detection limit: 0.3 ng/mL*
During the development phase, the analytical performance was performed on 2-fold serial dilutions of recNP-2.
The limit of detection (LOD) was defined as the last dilution that tested positive (15 tests, 2 independent readers).
Results may vary with recombinant protein, expression system, sequence, purification and storage
No cross-reaction with the following viruses:
- Influenza A
- Influenza B
- Respiratory Syncytial Virus (RSV)
- Respiratory Adenovirus
- Parainfluenza
- Rhinovirus
- Metapneumovirus
- Enterovirus
- Coronavirus HKU1
- Coronavirus OC43
- Coronavirus 229E
- Coronavirus NL63
ORIGIN
Kit components manufactured in Europe and the USA
The individual evaluation reports are available on the "Downloads" Tab
COV-Hygien Xpress DOCUMENTATION FOR DOWNLOAD
BROCHURE
Choose your language
PAPERS & REPORTS
White Papers, Study Reports
Environmental Testing for SARS-CoV-2 and the relevance of Immuno-Detection as a tool to contain spread of COVID-19
Title: Evaluation of COV-Hygien Xpress On-SiteDetection Kit
Topic: Spiking and sampling of SARS-CoV-2 protein on/from differentsurface types
Summary: Tested surfaces are metal, plastic and wood - LOD: 0,3-0,6 ng/10ul
By: CHROMagar
Title: Study of the use of a new immunochromatrographic test strip for Detection onsurfaces of protein specific to CORONAVIRUS SARS-CoV-2
Topic: Swabbing protocol evaluation, on different surfaces
Summary: Tested surfaces are metal, plastic, wood and ceramic - LOD: 2 ng ng/10ul except for wood (> 2ng)
By: QSA Conseil
Title: COV-Hygien Xpress Swabs
Topic: Evaluation of two swab types by sampling of SARS-CoV-2 protein from different surface types
Summary: Tested surfaces are metal, plastic and wood - LOD: 0,27-0,54 ng/10ul
By: CHROMagar
Title: Viral detection testing for surfaces
Topic: Spiking and sampling of SARS-CoV-2 protein on/from different surfaces
Summary: Tested surfaces are metal, plastic and glass - LOD: < 1 ng/10ul
By: Hylabs
Title: Data Sheet
Topic: Compilation of evaluation reports on kit detection limits
By: BioMire
PUBLICATIONS
Links to relevant scientific publications
Title: Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance
By: Daniel B. Larremore & Coll.
University of Colorado, Harvard University, Harvard T.H. Chan School of Public Health, Harvard Medical School, Howard Hughes Medical Institute
Title: Sustainability of Coronavirus on Different Surfaces
By: Rajiv Suman *, Mohd Javaid y, Abid Haleem y, Raju Vaishya z, Shashi Bahl x, Devaki Nandan.
University of Agriculture & Technology, Pantnagar, Indraprastha Apollo Hospital, I. K. Gujral Punjab Technical University
Title: Estimation of Viral Aerosol Emissions From Simulated IndividualsWith Asymptomatic to Moderate Coronavirus Disease 2019
By: Michael Riediker, Swiss Centre for Occupational and Environmental Health,Binzhofstrasse 87, CH-8404 Winterthur Switzerland
Title: Air and environmental sampling for SARS-CoV-2 around hospitalizedpatients with coronavirus disease 2019
By: Vincent Chi-Chung Cheng MD & Coll.
Department of Microbiology, Queen Mary Hospital, Hong Kong
Title: The effect of temperature on persistence of SARS-CoV-2 on common surfaces
By: Shane Riddell , Sarah Goldie, Andrew Hill, Debbie Eagles and Trevor W. Drew
Commonwealth Scientific and Industrial Research Organisation (CSIRO),Australian Centre for Disease Preparedness
INSTRUCTIONS FOR USE
Choose your language
APPLICATION NOTES
This application note introduces the use of COV-Hygien Xpress Control Protein as a positive control for the verification of the detection strips, the swabbing and the hygiene test protocol
MSDS (Material Safety Data Sheet)
WANT TO LEARN MORE ABOUT OUR COV-HYGIEN XPRESS KITS?
Visit our FAQ page or contact us.
We'd love to hear from you and answer any questions you may have!
A rapid, ready-to-use technology that detects SARS CoV-2 on surfaces
For use in:
- Offices and public buildings
- Hotels, restaurant, bars
- Airports, public transportation
- Clinics, ambulances
- Homes
Can be used to test:
- Doors, faucets, medical bag handles, handrails
- Light switches, control panels, keyboards
- Air vents
- Tables, desks, trays
- Telephones, garbage bins
Features
- Fast results
Results in under 15 minutes - Everything you need
Perform the test yourself on site without sending samples to a laboratory - Easy to use
No technical training required: as easy to use as
water quality test strips - Specific
Detects specific SARS CoV-2 proteins:
minimizes the risk of false positives - Economical
Inexpensive compared to PCR-based techniques
Benefits
- Reassures the public that surfaces have been tested for live virus and sanitized
- Improves sanitation procedures by incorporating routine testing for viral traces
- Immediate sanitization after a positive on-site test prevents further exposure to the virus
- Increases testing and monitoring data to support your sanitation procedures
WHAT DOES A POSITIVE OR NEGATIVE RESULT MEAN?
A positive test result indicates that the SARS-CoV protein N is present in the sample at a concentration greater than the detection limit.
A positive result does not indicate whether the virus is inactivated or infectious.
In the case of a hygiene test, a positive result indicates that the cleaning and disinfection procedure has failed to fulfill its purpose.
In the case of investigating surfaces suspected of contamination, a positive result confirms the recent presence of the virus.
In both cases, a positive result suggests that the sampled surface should be cleaned, sanitized, and retested.
SURFACE TESTING REASSURES YOURSELF AND OTHERS
Proper cleaning and sanitization are key to eliminating the threat of invisible COVID-19 contamination, particularly in highly frequented locations with "high-touch" surfaces. If you want to reassure members of the public who use your facility, your collaborators, and other stakeholders that your facility is safe, strengthen the impact of your sanitization procedures by demonstrating that they are effective.
The COV-Hygien Xpress test is a DIY kit that can be used by almost anyone, anytime, anywhere.
With results obtained in minutes, surface contamination can be addressed quickly, so everyone can relax.
READY TO GET STARTED?
ORDER YOUR COV-HYGIEN XPRESS KITS TODAY!
Get started right away, no specialized training or equipment required!
